Healthcare without the headache
See how to calm the chaos
Get same-day care
Compare optionsCare that takes you where you want to go
You’ve got the power to be better than yesterday. So get the care you want, the way you want it.
Preventive care
Personalized care for every health journey
Discover how our specialized services can help you get back to doing what you love.
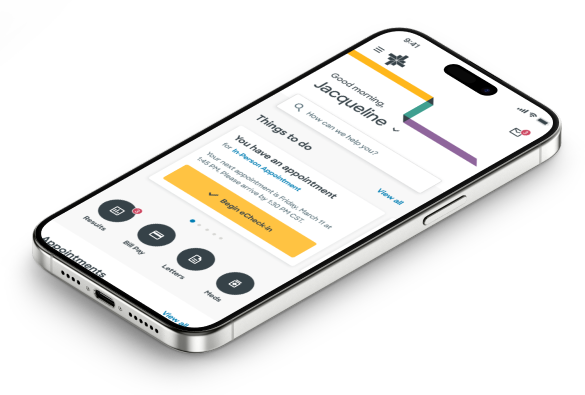
Let’s tailor your healthcare to you.
Use MyBSWHealth to get a personalized care experience.
Find It.
Fight It.

Find It.
Fight It.

Let’s shine a light on early cancer detection
The earlier you find it, the more power you have to fight it. And screenings are the best way to detect cancer early, even before you have visible symptoms. Check out the cleats we made for Dak Prescott that show how getting screened helps you see the unseen.
Get screenedGet the care you need. Like, fast.
If your back hurts, you should get it checked out. Together, we'll map out a clear path forward that keeps you in motion. It’s not complicated.
Your screenings aren’t just for you.
They’re for the whole crew. We make it easy to stay on top of your health so you can stay on track with your girls. Schedule today in our app.
Get care that keeps you on course.
Allergies got you down? You don’t have to jump through hoops to get the virtual care you need right now. It’s not complicated.
Getting headaches all the time? Get relief.
Get back to your happy place with a long-term plan that helps you keep your cool. It’s not complicated.



